Structure, Types and Working of Dry Cell

Working of Dry Cell
Catalog
Ⅰ Dry cell definition
A dry cell is an electrical power generator dependent on chemical reactions. The cell pushes the electrons to fluid from one end to the other when the two electrodes are bound in a closed way. The electron flow allows the electrons to flow into the closed system.
A dry cell battery is the easiest way of generating electricity. Several cells form a battery mixed together. The advanced variant of the dry cell is the lead-acid or nickel-cadmium battery. In the year 1866, the French engineer Georges Leclanche invented this cell. His invention was called Leclanche's battery by his name. But it was very heavy at the time and could easily be damaged. A dry cell comes in various voltages and sizes, and has the same concept, and is an improved variant of the Leclanche battery. Carl Gassner of Mainz invented the commercial shape of the zinc-carbon cell, a modified shape of the Leclanche battery, in 1881. It is manufactured in large quantities and used in many uses such as toys, radios, calculators, etc.

dry cell
The electrons migrate from one end to the other with the help of chemical reactions. If two or more cells are bound to proper polarity, more electrons are being circulated due to their high potential. The battery is called this mix. A battery can be used to achieve a range of voltages from a minimum voltage from 1.5 V to 100 V. Even the DC output voltage of a battery with power converters such as chopper circuits can be controlled to different levels.
Ⅱ Dry cell structure
The zinc-carbon dry cell structure can be seen in the figure. The anode terminal consists of a zinc or graphite rod as a general rule. The cathode terminal is petroleum. Zinc was used as the cathode, and graphite was used as an anode terminal in older models of dry cells. The element selection is focused primarily on its chemical configuration of the outermost orbit.

Dry cell symbol and structure
It can act as a donor and thus form a cathode if it has many electrons in its outermost orbit. In the same fashion, it can quickly accept and thus form the anode when the outermost orbit has fewer electrons. The interspersed electrolyte acts as a catalyst for chemical reactions. In general, we use ammonium chloride jelly as the electrolyte. The electrolyte is used as a zinc and chloride mixture in the figure shown. Often, the electrolyte also contains sodium chloride. The anode rod is surrounded by a mixture of manganese dioxide and carbon.
The entire structure is placed in a metal tube. By using a pitch on top of the cell, the gel is prevented from drying up. The bottom is a carbon washer. This washer is intended to avoid contact between the zinc anode rod and the bottle.
The diagram is also known as a spacer. The paper insulation can also cover the zinc for isolation. Other isolating materials like mica, etc. are also used for large batteries. On the top is the positive terminal of the ell. At the base is the negative end of the cell.
Ⅲ Dry cell types and working of dry cell
It can be graded as primary and secondary cells in accordance with the form of the dry cell. A primary cell is the one that cannot be reused or recharged. When all the chemical reagents are consumed by the electrochemical reactions, they do not generate power. On the other side, the battery charger can be used to refuel the chemical reactions by a secondary cell.
Primary cell
1. Zinc-Carbon cell
A dry cell consists of a container containing the graphite rod or a metal electron with a low moisture electrolyte paste. In general, a metal container is zinc, the base of which functions as a negative electron (anode), and a positive electrode is a carbon track (cathode). It is surrounded by manganese dioxide and low humidity electromagnetic components such as ammonium chloride paste that produce a maximum voltage of 1.5V and are not reversible.
The mechanism of half cell reaction has the following steps
1: During the process, the moisturized electrolyte consists of manganese (MnO2) and ammonium chloride (NH4Cl), a reduction reaction that supports the reductive reaction by graphit 1 1. 1: 1:
2NH4+ + 2MnO2 →Mn2O3 + 2NH3 + H2O
2: Zinc container acts as an anode and is oxidized.
Zn → Zn2+ + 2e-
The most common dry cell is Zinc-carbon and is also known as the Leclanche cell. The alkaline battery has almost same half-cell reaction, with the ammonium chloride replacing KOH or NaOH and the half-cell reactions
ZNCl2 + 2NH3 → Zn(NH3)2Cl2
2MnO2 + H2 → Mn2O3 + H2O
The overall reaction is,
Zn + 2MnO2 + 2NH4Cl → Mn2O3 + Zn(NH3)2Cl2 + H2O
2. Alkaline battery
The alkaline battery reactions are almost identical to those in zinc-carbon cells in which ammonium chloride and half-cell reactions are replaced by kOH or naOH.
Zn + 2OH– → ZnO + H2O + 2e–
2MnO2 + 2e– + H2O → Mn2O3 + 2OH–
3. Mercury cell
HgO acts as a cathode in a mercury cell, Zinc is an anode and the following steps take the reaction
1: At the anode
Zn + 2OH– → ZnO + H2O + 2e–
2: At the cathode
HgO + H2O + 2e– → Hg + 2OH–
The overall reaction is of the cell
Zn + HgO → ZnO + Hg
4. Silver oxide cell
In the fundamental medium, silver metal is an inert help in silver oxide reduction (Ag2O) and zinc oxidation.
1: Reaction at the cathode
Ag2O + 2H+ + 2e– → 2Ag + H2O
2: Reaction in the electrolyte
2H2O → 2H+ + 2OH–
3: Reaction at the anode
Zn + 2OH– → Zn(OH2) + 2e–
4: Overall reaction
Zn + H2O + Ag2O → Zn(OH2) + 2Ag
The overall reaction in an anhydrous medium
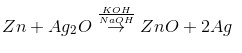
Secondary cell
1. Nickel-cadmium cell
Cadmium as an anode and Nickel as a cathode is nickel-cadmium cells and a separator serves as an isolator between the anode plates and the cathode plates. The electrolyte behaves as sodium hydroxide or potassium hydroxide.
Note: Cadmium is toxic to the atmosphere when disposed of. NiCd cells are also not in operation these days.
2. Lithium-ion cell
These batteries are common today on laptops, iPods, mobile devices. Lightweight carbon and lithium compose the electrodes of the cell. They are battery-low maintenance, and battery life requires no memory. Also after disposal, they are not less toxic, and less than half of the NiCd cell is self-discharge.
3. Nickel-metal hydride cell
NIMH serves as an anode and hydrogen-absorbing alloy acts as a cathode in the nickel-metal hydride cells. The Ni-MH battery's electrochemical characteristics are as follows
1: Reaction at the cathode
H2O + M + e– ⇋ OH– + MH
2: Reaction at the anode
Ni(OH)2 + OH– ⇋ NIO(OH) + H2O + e–
And at the end of the reaction nickel oxyhydroxide, NiO(OH) is formed.
Ⅳ Difference Between a Dry Cell and Wet Cell
The principal difference between the dry cell and the wet cell is the electromagnetic shape. As previously mentioned, the electrolyte, like ammonium chloride, is dry in nature in a dry cell. These dry cells are more frequent and are being used in toys, radios, etc. The electrolyte in a wet cell is, however, liquid.
Liquid electrolytes such as a toxic corrosive liquid called sulphuric acid are used. The water cell is more explosive in nature due to the nature of such liquids and must be treated with caution. They can be recharged and used for various applications to get the maximum advantage of such wet cells. Batteries such as these are commonly used in aviation, infrastructure, energy storage, and mobile towers.
Ⅴ Advantages and disadvantages of Dry cell
The dry cell has the following advantages:
The dry cell has many benefits, for example
The size of the house is tiny.
It can be supplied in different amounts of voltage.
It is practical and appliances are numerous.
It's the only DC voltage source.
It is possible to adjust the output voltage along with power electronic circuits
It can be rechargeable.
The disadvantages of the dry cell include the following:
It needs to be carefully treated
It is bright.
Batteries with a high rating are very strong
1.What is the function of a dry cell?
A dry cell has the electrolyte immobilized as a paste, with only enough moisture in it to allow current to flow. Unlike a wet cell, a dry cell can operate in any orientation without spilling, as it contains no free liquid. This versatility makes it suitable for portable equipment.
2.Where are dry cells used?
Leclanché battery, now called a dry cell, is produced in great quantities and is widely used in devices such as flashlights and portable radios.
3.What are dry cells and wet cells?
A wet-cell battery is the original type of rechargeable battery. A dry-cell battery does not contain liquid. Smaller dry-cell batteries, such as alkaline or lithium-ion, are typically used in portable electronics, such as toys, phones, and laptops.
4.Why is it called a dry cell?
Dry Cells. Many common batteries, such as those used in a flashlight or remote control, are voltaic dry cells. These batteries are called dry cells because the electrolyte is a paste. They are relatively inexpensive, but do not last a long time, and are not rechargeable.
5.What is inside a dry cell?
A dry cell is a type of electric battery, commonly used for portable electrical devices. A standard dry cell comprises a zinc anode, usually in the form of a cylindrical pot, with a carbon cathode in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc anode.
 Power Converter with Inbuilt Charging technology using WBG DevicesSaumitra Jagdale18 January 20242043
Power Converter with Inbuilt Charging technology using WBG DevicesSaumitra Jagdale18 January 20242043Although plug-in electric vehicles (PEVs) have gained traction globally, reducing cost, weight, and volume, while enhancing power conversion efficiency in chargers remains crucial. PEVs, recognized by governments for curbing fossil fuel consumption and emissions, rely on sizable battery packs for substantial all-electric range coverage.
Read More How to Choose the Best Deep Cycle Battery in 2024 | Reviews and Buying GuideUTMEL21 July 20252269
How to Choose the Best Deep Cycle Battery in 2024 | Reviews and Buying GuideUTMEL21 July 20252269You will learn about deep-cycle batteries in this post, including their main characteristics, things to think about while selecting one, and more.
Read More CR123A vs 123A Batteries Explained for 2025UTMEL27 May 2025963
CR123A vs 123A Batteries Explained for 2025UTMEL27 May 2025963CR123A and 123A batteries differ in capacity, lifespan, and compatibility. Learn how these differences affect your device's performance and safety.
Read More A Guide to the Best AG13 Battery SubstitutesUTMEL27 May 2025650
A Guide to the Best AG13 Battery SubstitutesUTMEL27 May 2025650Discover the top AG13 battery equivalents for 2025, including LR44, SR44, 357, and A76. Compare performance, compatibility, and cost for your devices.
Read More CR2450 vs CR2032 BatteriesUTMEL18 June 2025601
CR2450 vs CR2032 BatteriesUTMEL18 June 2025601CR2450 vs CR2032: CR2450 offers higher capacity and is larger, ideal for high-drain devices, while CR2032 suits compact, low-power gadgets. Learn more.
Read More
Subscribe to Utmel !
![DF22-3S-7.92C(28)]() DF22-3S-7.92C(28)
DF22-3S-7.92C(28)Hirose Electric Co Ltd
![SPUD-001T-P0.5]() SPUD-001T-P0.5
SPUD-001T-P0.5JST Sales America Inc.
![5025780300]() 5025780300
5025780300Molex
![FTSH-105-01-L-DV-K-TR]() FTSH-105-01-L-DV-K-TR
FTSH-105-01-L-DV-K-TRSamtec Inc.
![1200710045]() 1200710045
1200710045Molex
![DF11-2428SCFA]() DF11-2428SCFA
DF11-2428SCFAHirose Electric Co Ltd
![5015680707]() 5015680707
5015680707Molex
![S5B-ZR(LF)(SN)]() S5B-ZR(LF)(SN)
S5B-ZR(LF)(SN)JST Sales America Inc.
![5034290000]() 5034290000
5034290000Molex
![FH12A-30S-0.5SH(55)]() FH12A-30S-0.5SH(55)
FH12A-30S-0.5SH(55)Hirose Electric Co Ltd













