Introduction to Electrochemical Sensors
How do electrochemical-type sensors detect gas?
Catalog
Ⅵ Application of electrochemical sensors
| |
Ⅰ Introduction
Electrochemical sensors operate on the basis of ion conduction and chemical oxidation-reduction (redox) reactions. Based on their electrical output characteristics, electrochemical sensors can be classified into potentiometric sensors, conductometric sensors, amperometric sensors, polarographic sensors, and electrolytic sensors. These sensors are primarily used to analyze gas, liquid, or solid components dissolved in liquids, as well as for measuring liquid pH, conductivity, and oxidation-reduction potential (ORP).
With the rapid advancement of electronic technology and the Internet of Things (IoT), sensors based on electrochemical principles have become ubiquitous. They are widely used in the detection of harmful gases in the chemical industry, coal mining, environmental protection monitoring, and healthcare sectors. Because electrochemical sensors can respond selectively to a variety of toxic gases, possess a simple compact structure, and are relatively low-cost compared to infrared (NDIR) or PID technologies, they hold a dominant position in the safety detection of hazardous gases. Recent developments also include the integration of these sensors into wearable technology for non-invasive medical monitoring (e.g., glucose or lactate levels).
Ⅱ Principles of electrochemical sensors
An electrochemical sensor functions by allowing the target gas to enter the sensor housing and undergo an electrochemical reaction (oxidation or reduction) at the electrode surface. This reaction generates an electrical current (in amperometric sensors) or a potential difference (in potentiometric sensors) that is proportional to the gas concentration.
However, even when the sensor is in clean ambient air, the output signal is rarely absolute zero. This residual signal is known as the background current or baseline offset. This is a randomly changing signal influenced primarily by temperature fluctuations and, to a lesser extent, by the sensor's age. Occasionally, this value can be significant compared to the signal generated by low concentrations of the target gas. Therefore, eliminating the influence of background current—often through software algorithms or temperature compensation circuits—is crucial for improving measurement accuracy.
Modern portable gas detectors employ both hardware and software compensation. Hardware compensation involves adding specific thermistors or compensation circuits, while advanced Micro-Electro-Mechanical Systems (MEMS) sensors integrate ASIC chips to digitally correct these variances.
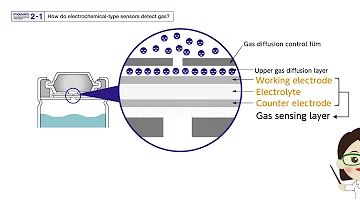
A typical electrochemical sensor consists of a Sensing (Working) Electrode and a Counter Electrode, separated by a thin layer of electrolyte.

Electrochemical sensor structure diagram
The gas first passes through a tiny capillary-shaped opening (which limits the flow rate to ensure diffusion control), then through a hydrophobic barrier layer, and finally reaches the electrode surface. This design allows the appropriate amount of gas to react with the sensing electrode to form a sufficient electrical signal while preventing the liquid electrolyte from leaking out.
The gas molecules reacting at the sensing electrode undergo either an oxidation mechanism (losing electrons) or a reduction mechanism (gaining electrons). These reactions are catalyzed by electrode materials—often precious metals like Platinum, Gold, or Carbon nanotubes—specifically selected for the target gas.
Through an external circuit connecting the electrodes, a current proportional to the gas concentration flows. Because the sensor generates its own current during this reaction, electrochemical sensors are often referred to as micro fuel cells.
In 2-electrode systems, polarization of the counter electrode can limit range and linearity. To improve performance, a third electrode, the Reference Electrode, is introduced. The reference electrode is installed in the electrolyte, adjacent to the sensing electrode but does not conduct current. Its sole purpose is to maintain a fixed, stable potential at the sensing electrode. A potentiostat circuit drives the counter electrode to balance the reaction without disturbing the reference potential. This 3-electrode architecture significantly improves linearity, range, and response time.
Ⅲ Composition of electrochemical sensors
The electrochemical sensor contains the following main components:

Electrochemical carbon monoxide gas sensor structure
A. Breathable Membrane (Hydrophobic Barrier): This membrane covers the sensing electrode. It is typically made of low-porosity PTFE (Teflon) or similar porous materials. Its primary function is to control the amount of gas molecules reaching the electrode (diffusion control) and to prevent the liquid electrolyte from leaking out. In "Capillary" type sensors, the gas flow is limited by a physical hole, while "Solid Membrane" types rely on diffusion through the material itself. The pore size must be optimized to allow gas entry while maintaining a liquid seal.
B. Electrodes: The choice of electrode material is critical for sensitivity and selectivity. Materials are typically precious metals (Platinum, Gold, Ruthenium) printed or deposited onto a substrate. Modern sensors utilize screen-printing technology to create robust electrode structures. The three electrodes (Working, Counter, and Reference) may use different catalysts depending on the chemical reaction required.
C. Electrolyte: The electrolyte facilitates ionic charge transfer between electrodes. Traditionally, these were liquid acids (e.g., Sulfuric acid) or bases. However, modern advancements have led to the use of Solid Polymer Electrolytes (SPE) and ionic liquids. SPE sensors are dry, eliminating the risk of leakage and allowing for thinner, more printable sensor form factors. If a liquid electrolyte evaporates (drying out), the sensor signal will drift and eventually fail.
D. Filter: To improve selectivity, a chemical scrubber filter is often placed in front of the sensor. For example, an activated carbon or permanganate filter might be used on a Carbon Monoxide (CO) sensor to remove Nitrogen Oxides or Alcohols that would otherwise cause a false positive reading. This is crucial for reducing cross-sensitivity.
Ⅳ Characteristics of electrochemical sensors

Electrochemical sensor
While manufacturing methods vary, the operational characteristics remain consistent across the technology:
1. Bias and Stabilization: Some sensors (like those for Nitric Oxide or Hydrogen Chloride) require a continuous bias voltage to keep the electrodes ready. Upon powering up or removing a shorting spring (jumper), these sensors may take 30 minutes to 24 hours to stabilize their baseline. Standard CO or H2S sensors usually do not require a bias and stabilize quickly.
2. Oxygen Requirement: Most toxic gas sensors function via oxidation and require a small amount of oxygen to complete the reaction at the counter electrode. In completely anaerobic environments, standard sensors may fail. Specialized "non-consumptive" or dual-chamber sensors are required for oxygen-free applications.
3. Humidity Influence: While the hydrophobic barrier blocks liquid water, water vapor can pass through. In high humidity, sensors can absorb water, potentially causing leakage (in liquid types). In extremely low humidity, liquid electrolytes can dry out, increasing internal resistance and slowing response time. Solid Polymer Electrolyte (SPE) sensors are significantly more resistant to these humidity extremes.
4. Cross-Sensitivity: Electrochemical sensors are not perfectly selective. For instance, a Carbon Monoxide sensor may also respond to Hydrogen or Ethylene. Users must consult the manufacturer's cross-sensitivity data table to understand potential interferences in their specific environment.
Ⅴ Advantages and disadvantages of electrochemical sensors
1. Advantages
Electrochemical sensors offer high accuracy and linearity for detecting toxic gases at low concentrations (parts per million or billion). They utilize very low power (microwatts), making them ideal for battery-operated portable detectors and wireless IoT nodes. The technology is mature, robust, and relatively low cost. Additionally, the development of Solid Polymer electrolytes has enabled screen-printed sensors that are smaller and more versatile for integration into consumer electronics.
2. Disadvantages
The primary disadvantage is lifespan. The chemical reagents and electrolytes are consumed or degraded over time. Typical sensors last 2 to 3 years, though modern Lead-Free Oxygen sensors and SPE sensors can achieve 5+ years. They are also sensitive to temperature changes, requiring compensation logic in the device. Finally, cross-sensitivity to non-target gases remains a challenge that requires careful filter selection or multi-sensor arrays to resolve.
Ⅵ Application of electrochemical sensors
1. Oxygen (O2) Sensor
The most common application of electrochemical technology is the Oxygen sensor. Unlike toxic gas sensors which operate amperometrically (generating current from zero), Oxygen sensors typically operate like a metal-air battery. As oxygen enters the sensor, it is reduced at the cathode, generating a current proportional to the oxygen concentration.
Traditionally, these sensors used a lead (Pb) anode which was consumed over time, limiting life to roughly 2 years. However, recent environmental regulations (RoHS) have driven the industry toward Lead-Free Oxygen sensors. These newer sensors utilize a non-consumptive reaction mechanism or "oxygen pump" principle, offering lifespans exceeding 5 years and reducing environmental waste. They are critical for confined space entry, diving equipment, and medical anesthesia monitoring.
2. Nitrogen oxide sensor
Nitrogen oxides (NOx) are a mixture of gases, primarily Nitric Oxide (NO) and Nitrogen Dioxide (NO2), which are major pollutants from combustion engines and industrial processes. In environmental analysis, monitoring these at low levels is crucial. While traditional methods involve chemiluminescence, electrochemical sensors offer a portable solution. Literature reports new types of gas-sensitive microsensors combining microelectronic integrated circuits with chemically active films, such as Copper Phthalocyanine, which can selectively detect mg/m³ levels of Nitrogen Dioxide. These sensors are vital for air quality monitoring in smart cities.
3. Hydrogen sulfide gas sensor
Hydrogen sulfide (H2S) is a colorless, combustible gas with a distinct "rotten egg" odor. It is highly toxic and commonly found in sewers, oil & gas fields, and wastewater treatment plants. Electrochemical H2S sensors are the industry standard for personal safety monitors. Unlike semiconductor sensors, electrochemical variants offer excellent linearity and are less prone to false alarms from humidity. Advanced sensor arrays allow for the simultaneous recording of Sulfur Dioxide and Hydrogen sulfide, distinguishing between these cross-sensitive gases.

Electrochemical hydrogen sulfide sensor
4. Sulfur dioxide sensor
Sulfur dioxide (SO2) is a significant air pollutant resulting from burning fossil fuels containing sulfur. Monitoring SO2 is a standard requirement for industrial emissions and urban air quality. Electrochemical SO2 sensors utilize an oxidation reaction (typically at 0.65V bias). These sensors exhibit high current sensitivity, fast response times (< 20 seconds), and low background noise. Modern designs using organic modified silicate thin films via sol-gel processes have improved the stability and reproducibility of these sensors. Crucially, these sensors have low cross-sensitivity (interference) and are robust against temperature and humidity fluctuations compared to older technologies.
Article Recommended:
What is electrochemical sensor?
Electrochemical sensors are devices that give information about the composition of a system in real time by coupling a chemically selective layer (the recognition element) to an electrochemical transducer.
How do electrochemical sensors work?
To put things simply, this sensor type works by means of gas diffusion. Gas finds its way into the outlet of the membrane on top of the sensor housing. Once the gas reaches the working electrode, an electrochemical reaction occurs.
What is electrochemical sensors used for?
Electrochemical sensors are used for detecting oxygen and toxic gases. More specifically, they measure the concentration of a specific gas within an external circuit. This is done by method of oxidation or reduction reactions.
What is the principle of operation of electrochemical sensing portable instruments?
Electrochemical sensors are operated based on the diffusion of gas of interest into the sensor, which results in the production of an electrical signal that is proportional to the gas concentration.
 The Key Role of Electronic Components in IoT DevicesUTMEL01 September 20234974
The Key Role of Electronic Components in IoT DevicesUTMEL01 September 20234974The article discusses the pivotal role of electronic components in Internet of Things (IoT) devices. IoT devices work by capturing real-world data using sensors, processing it through a microcontroller, and then sending it to the cloud for further analysis.
Read More How to Identify the Perfect Proximity Sensor for Your ApplicationUTMEL19 July 2025881
How to Identify the Perfect Proximity Sensor for Your ApplicationUTMEL19 July 2025881Find the best proximity sensors for your project by evaluating material, sensing range, environment, and system needs to ensure optimal performance and reliability.
Read More Trusted Vibration Sensors for Homeowners and Industry ProfessionalsUTMEL17 July 2025591
Trusted Vibration Sensors for Homeowners and Industry ProfessionalsUTMEL17 July 2025591Compare top vibration sensors for home and industrial use. Find trusted options for security, predictive maintenance, and equipment protection.
Read More Wiring and Mounting Photoelectric Sensors in 2025UTMEL15 July 2025789
Wiring and Mounting Photoelectric Sensors in 2025UTMEL15 July 2025789Wire and mount photoelectric sensors in 2025 with step-by-step safety, wiring, and alignment tips for reliable installation and optimal sensor performance.
Read More Essential Tips for Picking the Best Gas SensorUTMEL15 July 20251867
Essential Tips for Picking the Best Gas SensorUTMEL15 July 20251867Find out how to select gas sensors by matching target gases, environment, and compliance needs for reliable and accurate gas detection in any setting.
Read More
Subscribe to Utmel !





